Imaging Facility
The shared Boston College imaging facility has 712 sq. ft. of space within Higgins Hall, the physics and biology building completed in 2002 on the main campus of BC. The facility has five separate spaces for microscopes and office space for the facility director (Bret Judson), which also houses several workstations for image analysis by facility users. Bench space is available for set up of experiments and a fume hood is present within the facility.
Acknowledgement of Core Facility Document

Axioplan 2. Zeiss Axioplan 2: The Axioplan 2 imaging is a universal upright microscope which is used for light (transmitted and incident) and fluorescence microscopy. Fluorescence illumination is provided by a Lumencor SOLA light engine. This light engine is more user and environmentally friendly by eliminating the need for a mercury lamp. The microscope is controlled by Micromanager software (operating a cooled ccd black/white Hamamatsu camera) and allows collection of time-lapse images in both DIC and fluorescent channels (DAPI, FITC, TRITC). An objective heater ensures tissue culture cell integrity during time-lapse experiments. In addition, the microscope is equipped with a digital color camera (SPOT, Insight) enabling collection of stained histology tissue sections.

Axioimager Z2. This is an upright microscope outfitted for both transmitted (including DIC, and phase) and fluorescence imaging. Fluorescence illumination is provided by an EXFO LED light source eliminating the mercury lamp. It is fully automated allowing acquisition in multiple X, Y and Z positions. The microscope is equipped with objectives from 5x to 100x. Image capture and analysis is through the Zeiss ZEN software. For large data sets there is an off-line image analysis workstation running ZEN.
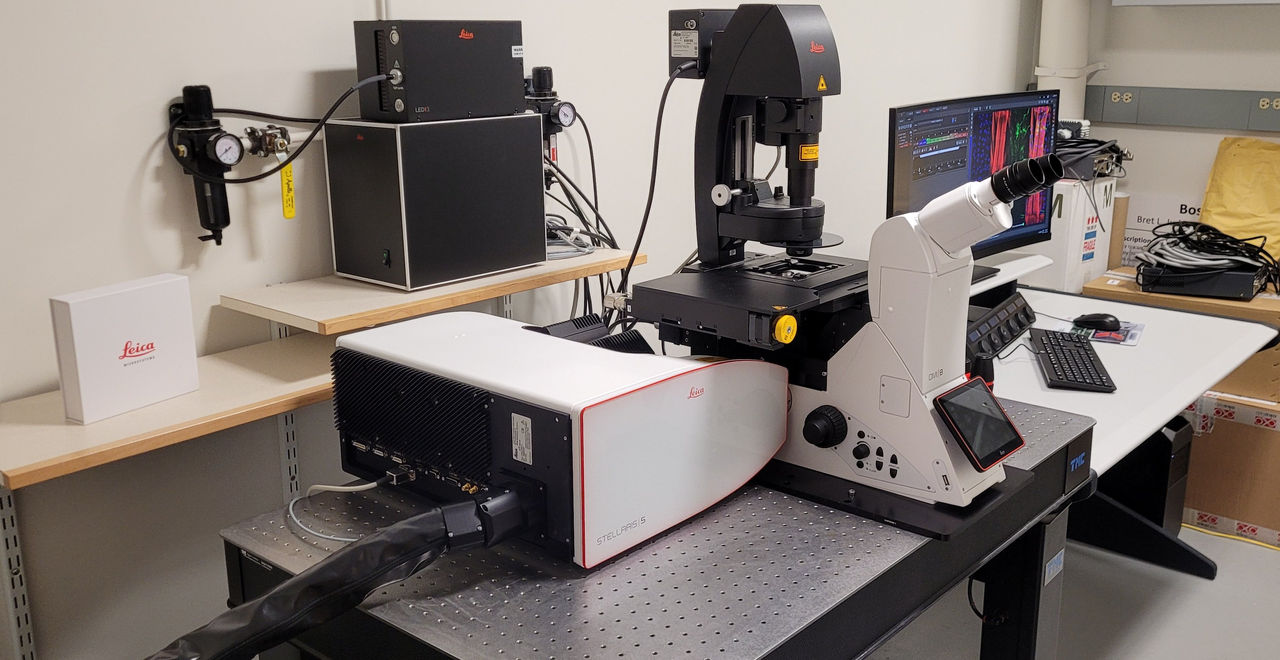
Leica Stellaris 5. Leica Stellaris 5 laser scanning confocal microscope: This system is ideal for 3D imaging of UV and visible fluorophores (4 colors) in fixed or live samples. This system combines transmitted light techniques with confocal imaging for a highly flexible platform. The Leica software running this system allows for time lapse, Z Stacks, large area tiling, and 3D reconstruction. For better than standard resolution the system is also equipped with Leica’s Lightning module.

Zeiss LSM 880-AS FAST Elyra.S1: This system combines several technologies in to one flexible platform. At the base level the system is the latest confocal microscope from Zeiss capable of imaging samples at multiple wavelengths from 405nm to 633nm and from magnifications of 10x to 100x. Included on the system are two “super-resolution” modalities – the Airyscan/FAST unit and the Elyra unit. The Airyscan technology improves resolution down to 140 nm laterally and 400 nm axially. In addition the Airyscan module can be run in the “FAST” mode for live samples. For users requiring better resolution than the Airyscan can provide the system has the Elyra unit which can provide resolution of 120nm laterally and 300nm axially. Depending on experimental needs users can freely change between any of these technologies. For samples requiring temperature control the system is outfitted with both heating and cooling inserts.
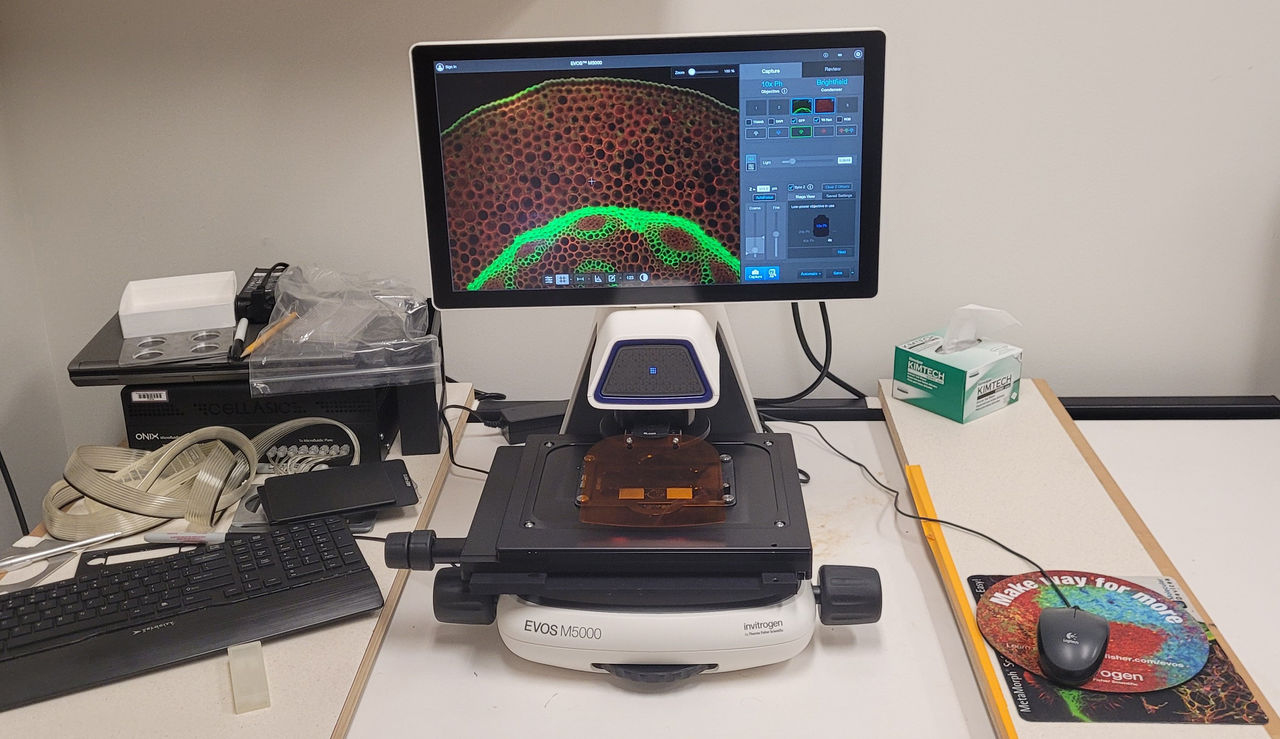
EVOS M5000. Life Technologies EVOS M5000: This is an inverted microscope that allows imaging in a variety of configurations from slides to 96-well plates. The microscope is equipped with a variety of dry objectives and includes phase contrast. The stage is motorized in Z allowing for easy focus adjustments and Z stack capabilities. Illumination is all LED eliminating the mercury lamp traditionally used for fluorescence illumination.

Spinning Disk. 3i (Intelligent Imaging Innovations) spinning disk confocal: The facility houses an Intelligent Imaging Innovations (3I) integrated Yokogawa spinning disk confocal on a Zeiss Axio Observer fully automated inverted microscope. The spinning disk system includes: 4 laser lines, a Prime 95B sCMOS camera (Photometrics), temperature controlled stage adapter (Okolab), the Vector2 system (3I) for photokinetic experiments and the Ablate module (3I) for targeted wounding in cells and tissues.
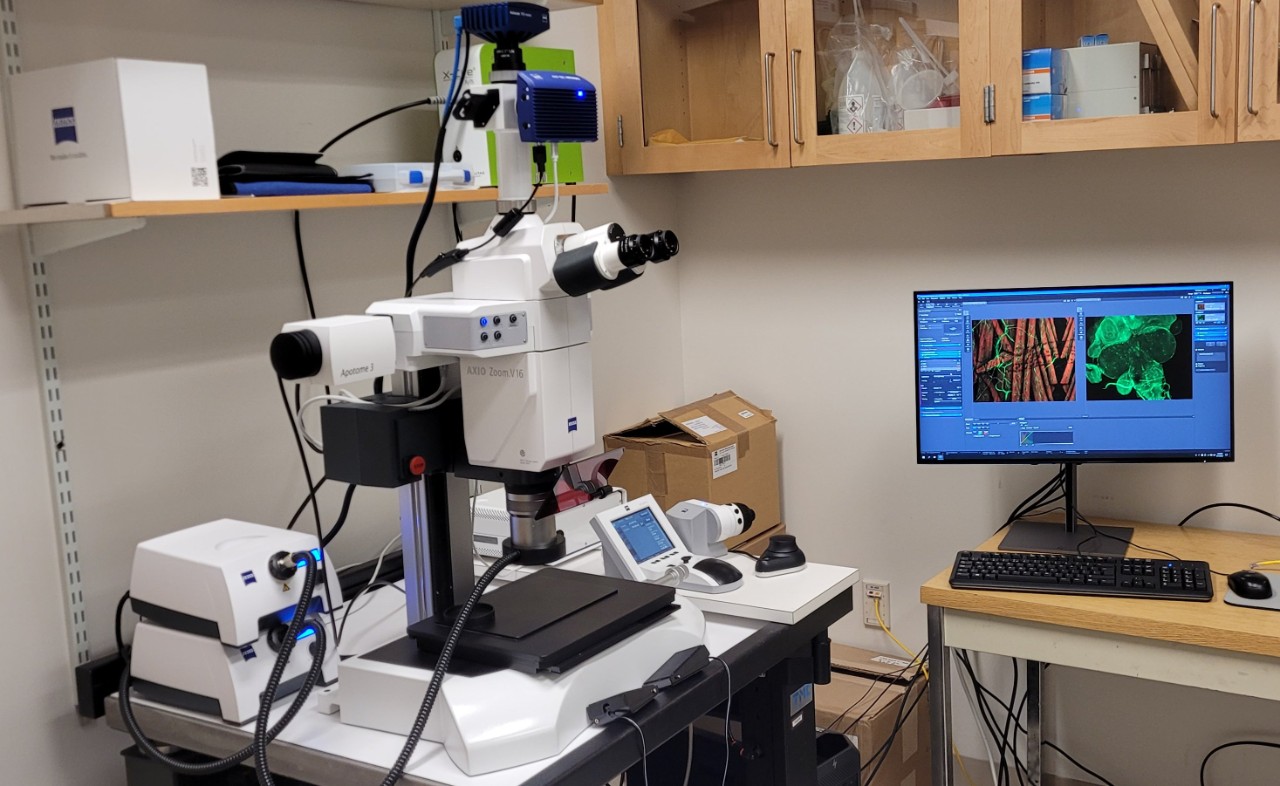
Zeiss Axio Zoom V16. This is a stereo microscope with a 16x zoom range. It is equipped with both a color camera for transmitted/reflected light imaging and a monochrome camera for fluorescence imaging with 4 fluorescent cubes (DAPI, GFP, RFP and CY5). Additionally, the microscope is equipped with the Apotome.3 for optical sectioning when using fluorescence. It is fully motorized allowing acquisition of a large Z range. Image capture and analysis is through the Zeiss ZEN software. This microscope is ideally suited for imaging large specimens at high resolution. For large data sets there is an off-line image analysis workstation running ZEN.
Workstation. Workstation with image analysis software: There is an off-line image analysis computer running both Windows and Mac OS. This computer can be used for any off-line image analysis including Leica LAS, Fiji, and Prism software.
The microscopes are located in room 525. For all microscope questions and training, please contact: Bret Judson.
Facility Documentation
Guidelines for Bringing Live Samples into the Imaging Facility
Acknowledgement of Core Facility Document
Please note that all work performed in BC core facilities and recharge centers should always be appropriately acknowledged. If you are publishing or presenting data acquired in BC core facilities and recharge centers, please include the following statement in the Acknowledgement section of your manuscript/poster/presentation, "The authors would like to thank the Boston College <insert facility name> for assistance with the work presented in this paper/poster/presentation*."
* Delete as appropriate