By “erasing” and rebuilding the receptor-binding ability of a virus viewed as the prime vehicle for new human gene therapies, a team of Boston College researchers has engineered a tougher, more selective version of the virus capable of attacking cancer cells.
The team, led by Assistant Professor of Chemistry Abhishek Chatterjee and graduate student Rachel Kelemen, developed a novel chemical strategy to precisely introduce synthetic receptor-binding agents onto the fragile casing of the virus in order to pursue malignant cells with an unprecedented selectivity, according to the team’s most recent research, published in the international edition of the German journal Angewandte Chemie.
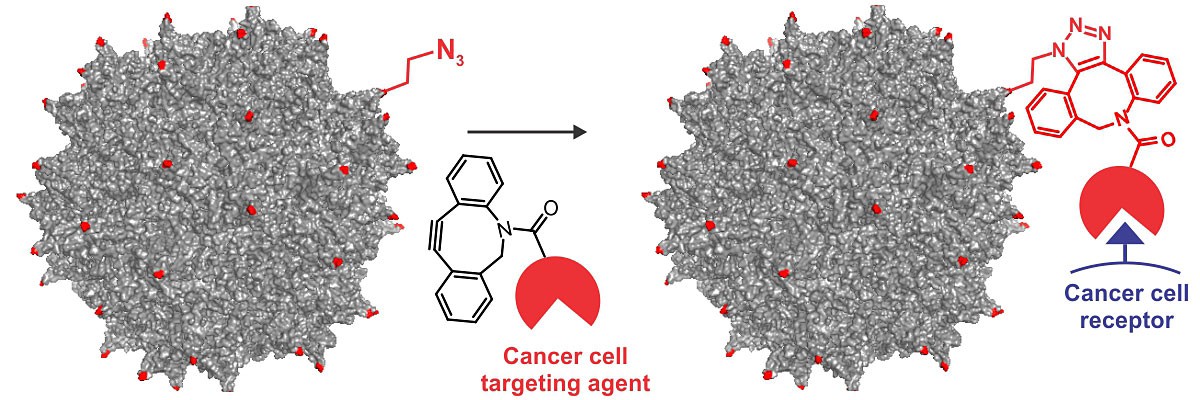
“Viruses have perfected the art of invading human cells and delivering their genetic material,” said Chatterjee, a chemist who focuses on engineering complex biological systems. “However, the delicate virus capsids do not generally tolerate large modifications, rendering such engineering efforts challenging. Using adeno-associated virus – the most promising candidate for human gene therapy – we have developed a new approach to engineer its infectivity.”
Adeno-associated virus attaches to a cell by binding specific surface-exposed receptor proteins. Chatterjee said the lab targeted that ability by wiping clean the virus’ native binding capacity and replacing it with a synthetic binding agent tailored to a receptor on the target cell.
“We developed a novel chemical strategy to precisely introduce such synthetic receptor-binding agents onto the virus capsid with unprecedented site-selectivity,” said Chatterjee, who co-authored the report “A Precise ChemicalStrategy To Alter the Receptor Specificity of the Adeno-Associated Virus” with Kelemen and BC researchers Raja Mukherjee, Xiaofu Cao, and Sarah B. Erickson.
To enable site-specific virus engineering, Chatterjee said the team first developed a method to incorporate small unnatural amino acid building blocks into specific sites of the virus capsid, without damaging the fragile structure. The approach succeeded in providing attachment sites for synthetic binding agents through a highly selective chemical conjugation reaction.

Using the new strategy, Chatterjee attached a small cancer cell-targeting ligand to an adeno-associated virus, which lacks the natural ability to bind its native receptor. Experiments found the virus infected cancer cells with a high degree of specificity, validating the team’s site-selective approach to virus engineering.
Chatterjee said the approach can be applied to enable the virus to specifically target many kinds of human cells.
“Our technology for the first time enables site-specific chemical modification of adeno-associated virus,” said Chatterjee. “While not unexpected, it was gratifying to demonstrate that the ability to control the attachment site on the virus capsid is critical to generate functional virus-targeting agent conjugates that efficiently and selectively infect a desired cell population.”
Chatterjee said he and researchers in his lab are now trying to develop the approach to introduce a toxic gene specifically into cancer cells in order to induce cell death. In addition, Chatterjee is applying the technique to generate more efficient adeno-associated virus delivery systems that can introduce therapeutic genes into target cells in order to cure diseases such as blindness, deafness, hemophilia and other genetic disorders.
“The ability to reprogram a virus to specifically infect a target cell population and deliver a customized genetic cargo, will lead to the generation of novel therapeutic strategies for supplementing a necessary gene into deficient cells, or delivering a toxic gene to malignant cells,” Chatterjee said. “We’re glad that our research has advanced the capacity of scientists to push closer to these new approaches.”
-Ed Hayward / University Communications