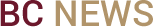Sheehan, in his laboratory in Fall River, Massachusetts. (Photo by Gary Wayne Gilbert)
A 29-year-old electrochemist and entrepreneur, Stafford Sheehan in 2015 founded Catalytic Innovations, a Rhode Island-based company whose goal, he says, is “to try and save the world, basically”; to fight climate change and eventually bypass fossil fuels; to take carbon dioxide from the air and, using solar-powered electricity, transform it into fuels and other commercial chemicals. And, crucially, to develop technology that can do all that inexpensively enough and on a large enough scale to be commercially viable. That last part has eluded scientists for years, despite the promise that artificial photosynthesis has shown in the laboratory.
Sheehan, who built the company’s first reactor in his mother’s garage and has since taken on two full-time employees and two cofounders—including Yale chemist Paul Anastas, who led the EPA’s research and development office under Obama—has had some success already, both scientifically and commercially. Right now, he is working to scale up his production of ethanol, which is used not only as a biofuel but also in fragrances, cleaners, and alcoholic spirits. “We can make ethanol that is cleaner and purer and more renewable,” Sheehan says, “than what you get from fermenting corn,” which requires distilling out toxic compounds such as methanol.
Meanwhile, Sheehan has found industrial applications—and paying customers—for individual parts of his technology. Iridium-based catalysts discovered early in his research on artificial photosynthesis can be used on their own to prevent lead from leaching into wastewater during metal refining processes. His catalysts also help treat dairy wastewater, converting lactic acid, a contaminant created in Greek yogurt production, into carbon dioxide. In 2016, Forbes named Sheehan to its list of “30 Under 30” in the energy field, and earlier this year Chemical and Engineering News included him as one of 2017’s “Talented Twelve.”
Originally a computer science major and Arabic minor at Boston College (and then, briefly, pre-med), Sheehan took a chemistry class his freshman year that led pretty quickly to a job in associate professor Dunwei Wang’s lab, researching ways to use metal oxides—rust, for example—to split water into hydrogen (a fuel) and oxygen, with sunlight. He went on to Yale and a Ph.D. in physical chemistry. “The idea was always to make a renewable fuel—carbon-neutral with sunlight as the input,” he says. “I started doing that in Dunwei’s lab and never stopped.”
Lydialyle Gibson is a writer in the Boston area.